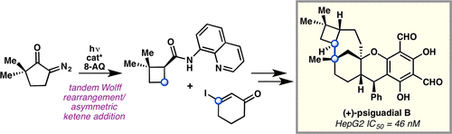
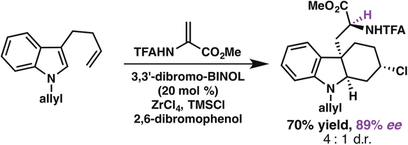
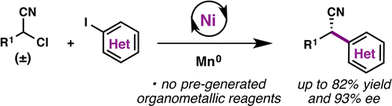
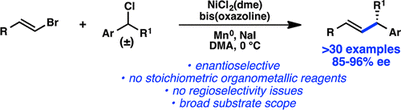
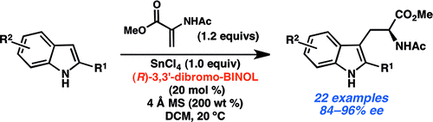
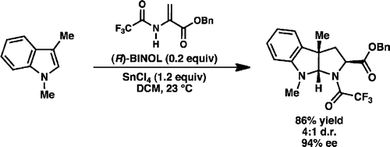
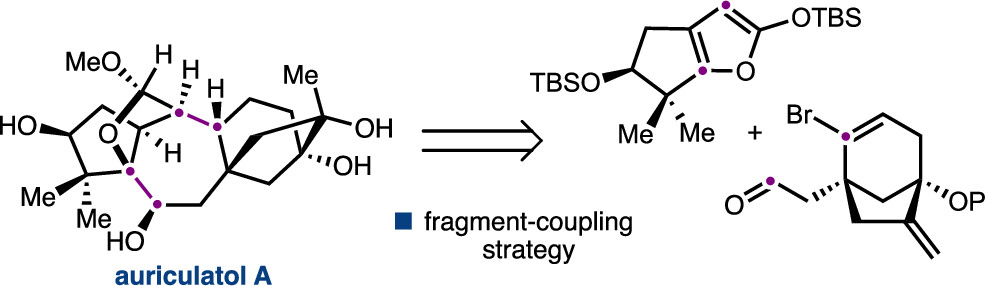
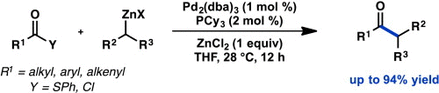92. Enantioselective Synthesis of (+)-Auriculatol A. Thompson J. K., Youngblood K. C., Teh S., Farley C. M., Zhang Z., Virgil S. C., Reisman S. E. J. Am. Chem. Soc., 2025, 147, 46, 42170–42174. DOI: 10.1021/jacs.5c17269
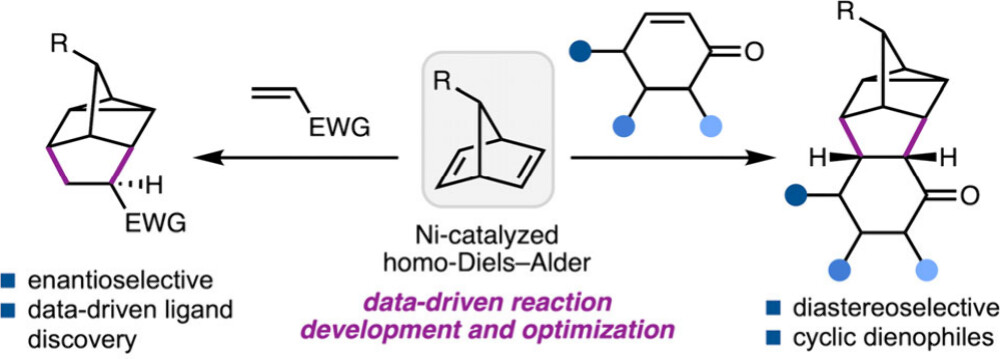
91. A Data Science-Guided Approach for the Development of Nickel-Catalyzed Homo-DielsAlder Reactions. Cadge J. A.; Lozano C.; Merriman M. T.; Oblad P; Sigman M. S.; Reisman S. E. J. Am. Chem. Soc., 2025, 147, 34, 31175–31186. DOI: 10.1021/jacs.5c09948
90. Designing Target-specific Data Sets for Regioselectivity Predictions on Complex Substrates. Schleinitz J.; Carretero-Cerdán A.; Gurajapu A.; Harnik Y.; Lee G.; Pandey A.; Milo A.; Reisman S. E. J. Am. Chem. Soc., 2025, 147, 9, 7476-7484. DOI: 10.1021/jacs.4c15902
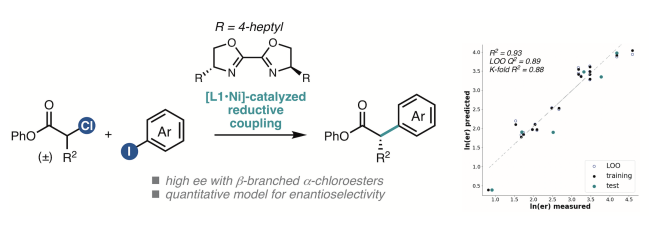
89. Ni-Catalyzed Enantioselective Desymmetrization: Development of Divergent Acyl and Decarbonylative Cross-Coupling Reactions. Hernández-Mejías A. D.; Shimozono A. M.; Hazra A.; Richter S.; Tong Z.; Langille N. F.; Quasdorf K.; Parsons A. T.; Sigman M. S.; Reisman S. E. J. Am. Chem. Soc., 2025, 147, 4, 3468-3477. DOI: 10.1021/jacs.4c14767
88. Ni-Catalyzed Asymmetric Reductive Arylation of α-Substituted Imides. Chen L. M.; Shin C.; DeLano T.; Carretero-Cerdán A.; Gheibi G.; Reisman S. E. J. Am. Chem. Soc., 2024, 146, 43, 29523-29530. DOI: 10.1021/jacs.4c09327
87. Reductive Samarium (electro)catalysis Enabled by SmIII-alkoxide protonolysis. Boyd E. A.†; Shin C.†; Charboneau D. J.; Peters J. C.; Reisman S. E. Science, 2024, 385, 6711, 847–853. DOI: 10.1126/science.adp5777
86. Enantioselective C(sp2)–C(sp3) Bond Construction by Ni Catalysis Chen L. M.; Reisman S. E. Acc. Chem. Res., 2024, 57, 5, 751-762. DOI: 10.1021/acs.accounts.3c00775
85. Electrocatalytic Asymmetric Nozaki–Hiyama–Kishi Decarboxylative Coupling: Scope, Applications, and Mechanism Gao Y.; Jiang B.; Friede N. C.; Hunter A. C.; Boucher D. G.; Minteer S. D.; Sigman M. S.; Reisman S. E.; Baran P. S. J. Am. Chem. Soc., 2024, 146, 7, 4872-4882. DOI: 10.1021/jacs.3c13442
84. Dataset design for building models of chemical reactivity. Raghavan P.; Haas B. C.; Ruos M. E.; Schleinitz J.; Doyle A. G.; Reisman S. E.; Sigman M. S.; Coley C. W. ACS Cent. Sci. 2023, 9, 12, 2196–2204. DOI: 10.1021/acscentsci.3c01163
83. A Pyridine Dearomatization Approach for the Gram Scale Synthesis of ()-Sparteine. Lam, P. H.; Kerkovius, J. K.; Reisman, S. E. Org. Lett. 2023, 25, 46, 8230-8233. DOI: 10.1021/acs.orglett.3c03242
82. Isolation, biosynthesis, and chemical syntheses of the hasubanan and acutumine alkaloids: A historical perspective. Grünenfelder, D. C.; Reisman, S. E.; Navarro, R. Tetrahedron. 2023, 149, 133709. DOI: 10.1016/j.tet.2023.133709
81. Development of a Nickel-Catalyzed N–N Coupling for the Synthesis of Hydrazides. Barbor, J. P.; Nair, V. N.; Sharp, K. R.; Lohrey, T. D.; Dibrell, S. E.; Shah, T. K.; Walsh, M. J.; Reisman, S. E.; Stoltz, B. M. J. Am. Chem. Soc. 2023, 145, 28, 15071. DOI: 10.1021/jacs.3c04834
80. Mechanistic Investigation of Ni-Catalyzed Reductive Cross-Coupling of Alkenyl and Benzyl Electrophiles. Turro, R. F.; Wahlman, J. L. H.; Tong, Z. J.; Chen, X.; Yang, M.; Chen, E. P.; Hong, X.; Hadt, R. G.; Houk, K. N.; Yang, Y.; Reisman, S. E. J. Am. Chem. Soc. 2023, 145, 27, 14705. DOI: 10.1021/jacs.3c02649
79. Electronic Structures of Nickel(II)-Bis(indanyloxazoline)-dihalide Catalysts: Understanding Ligand Field Contributions that Promote C(sp2)–C(sp3) Cross-Coupling. McNicholas, B. J.; Tong, J.; Bím, D.; Turro, R. F.; Kazmierczak, N. P.; Chalupský, J.; Reisman, S. E.; Hadt, R. G. Inorg. Chem. 2023, 62, 34, 14010-14027. DOI: 10.1021/acs.inorgchem.3c02048
78. Electrochemical Preparation of Sm(II) Reagent Facilitated by Weakly Coordinating Anions. Ware, S. D.; Zhang, W.; Charboneau, D. J.; Klein, C. K.; Reisman, S. E.; See, K. A. Chem. Eur. J. 2023, 29, e202301045. DOI: 10.1002/chem.202301045
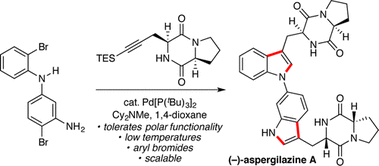
77. A convergent fragment coupling strategy to access quaternary stereogenic centers. Kerkovius, J. K.; Wong, A. R.; Mak, V. W.; Reisman, S. E. Chem. Sci. 2023, 14, 16, 4397-4400. DOI: 10.1039/D2SC07023E
76. Enantioselective Synthesis of N-Benzylic Heterocycles by Ni/Photoredox Dual Catalysis. Lacker, C. R.; Delano, T. J.; Chen, E. P.; Kong, J.; Belyk, K. M.; Piou, T.; Reisman, S. E. J. Am. Chem. Soc. 2022, 144, 20190. DOI: 10.1021/jacs.2c07917
75. A Pyridine Dearomatization Approach to the Matrine–Type Lupin Alkaloids. Kerkovius, J. K.; Stegner, A.; Turlik, A.; Lam, P.H.; Houk, K.N.; Reisman, S. E. J. Am. Chem. Soc. 2022, 144, 15938. DOI: 10.1021/jacs.2c06584
74. Expanding the Chiral Monoterpene Pool: Enantioselective Diels–Alder Reactions of α–Acyloxy Enones. Mendoza, S. D.; Rombola, M.; Tao, Y.; Zuend, S. J.; Götz, R.; McLaughlin, M. J.; Reisman, S. E. Org. Lett. 2022, 24, 3802. DOI: 10.1021/acs.orglett.2c01343
73. Synthesis of Noraugustamine and Development of an Oxidative Heck/Aza-Wacker Cascade Cyclization. Holman, K. R.; Stanko, A.M.; Richter, M.J.R.; Feng, S. S.; Gessesse, M. N.; Reisman, S. E. Org. Lett. 2022, 24, 3019. DOI: 10.1021/acs.orglett.2c00948
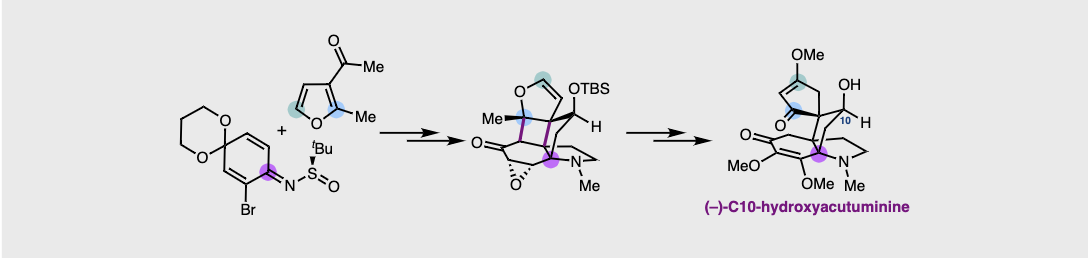
72. Enantioselective Synthesis of (–)-C10-Hydroxyacutuminine. Grünenfelder, D. C.; Navarro, R.; Wang, H.; Fastuca, N. J.; Butler, J. R.; Reisman, S. E. Angew. Chem. Int. Ed. 2022, 62, e202117480. DOI: 10.1002/anie.202117480
71. Plugging the Leak: Empowering Women in Organic Chemistry. Dibrell, S. E.; Holman, K. R.; Reisman, S. E. Angew. Chem. Int. Ed. 2022, 61, e202111765. DOI: 10.1002/anie.202111765
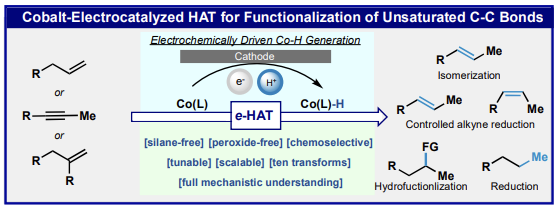
70. Cobalt-Electrocatalytic Hydrogen Atom Transfer for Functionalization of Unsaturated C–C Bonds. Gnaim, S.; Bauer, A.; Zhang, H.-J.; Chen, L.; Gannet, C.; Malapit, C. A.; Hill, D. E.; Vogt, D.; Tang, T.; Daley, R.; Hao, W.; Quertenmont, M.; Beck, W. D.; Kandahari, E.; Vantourout, J. C.; Echeverria, P.-G.; Abruna, H.; Blackmond, D. G.; Minteer, S.; Reisman, S. E.; Sigman, M. S.; Baran, P. S. Nature 2022, 605, 687. DOI: doi.org/10.1038/s41586-022-04595-3
69. 3D Computer Vision Models Predict DFT-Level HOMO-LUMO Gap Energies from Force-Field-Optimized Geometries. Maser, M. R.; Reisman, S. E. ChemRxiv
68. Nickel-Catalyzed Reductive Alkylation of Heteroaryl Imines. Turro, R. F.; Brandstätter, M.; Reisman, S. E. Angew. Chem. Int. Ed. 2022 e202207597 DOI:10.1002/anie.202207597
67. Electrochemical Nozaki–Hiyama–Kishi Coupling: Scope, Applications, and Mechanism. Gao, Y.; Hill, D. E.; Hao, W.; McNicholas, B. J.; Vantourout, J. C.; Hadt, R. G.; Reisman, S. E.; Blackmond, D. G.; Baran, P. S. J. Am. Chem. Soc. 2021, 143, 9478. DOI: 10.1021/jacs.1c03007
66. Total Syntheses of the C19 Diterpenoid Alkaloids (–)-Talatisamine, (–)-Liljestrandisine, and (–)-Liljestrandinine by a Fragment Coupling Approach. Wong, A. R.; Fastuca, N. J.; Mak, V. W.; Kerkovius, J. K.; Stevenson, S. M.; Reisman, S. E. ACS Cent. Sci. 2021, 7, 1311. DOI: 10.1021/acscentsci.1c00540.
65. Palladium-Catalyzed Cascade Cyclizations Involving C–C and C–X Bond Formation: Strategic Applications in Natural Product Synthesis. Holman, K. R.; Stanko, A. M.; Reisman, S. E. Chem. Soc. Rev. 2021, 50, 7891. DOI: 10.1039/D0CS01385D
64. Total Synthesis of Complex Natural Products: More Than a Race for Molecular Summits. Reisman, S. E.; Maimone, T. J. Acc. Chem. Res. 2021, 54, 1815. DOI: 10.1021/acs.accounts.1c00184
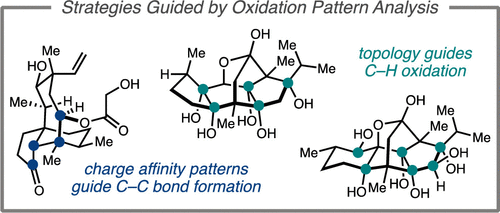
63. Synthesis of Complex Diterpenes: Strategies Guided by Oxidation Pattern Analysis. Dibrell, S. E.; Tao, Y.; Reisman, S. E. Acc. Chem. Res. 2021, 54, 1360. DOI: 10.1021/acs.accounts.0c00858
62. Total Synthesis of Ritterazine B. Nakayama, N.; Maser, M. R.; Okita, T.; Dubrovskiy, A. V.; Campbell, T. L.; Reisman, S. E. J. Am. Chem. Soc. 2021, 143, 4187. DOI: 10.1021/jacs.1c0137261. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling of a-Chloroesters with (Hetero)Aryl Iodides. DeLano, T. J.; Dibrell, S. E.; Lacker, C. R.; Pancoast, A. R.; Poremba, K. E.; Cleary, L. B.; Sigman, M. S.; Reisman, S. E. Chem. Sci. 2021, 12, 7758. DOI: 10.1039/D1SC00822F
60. Multi-Label Classification Models for the Prediction of Cross-Coupling Reaction Conditions. Maser, M. R.; Cui, A. Y.; Ryou, S.; DeLano, T. J.; Yue, Y.; Reisman, S. E. J. Chem. Inf. Model. 2021, 61, 156. DOI: 10.1021/acs.jcim.0c01234
59. Asymmetric Michael Addition of Dimethyl Malonate to 2-Cyclopenten-1-one Catalyzed by a Heterobimetallic Complex. Fastuca, N. J.; Wong, A. R.; Mak, V. W.; Reisman, S. E. Org. Synth. 2020, 97, 327. DOI: 10.15227/orgsyn.097.0327
58. A Copper-Catalyzed Asymmetric Oxime Propargylation Enables the Synthesis of the Gliovirin Tetrahydro-1,2-oxazine Core. Cowper, N. G. W.; Hesse, M. J.; Chan, K. M.; Reisman, S. E. Chem. Sci. 2020, 11, 11897. DOI: 10.1039/D0SC04802J
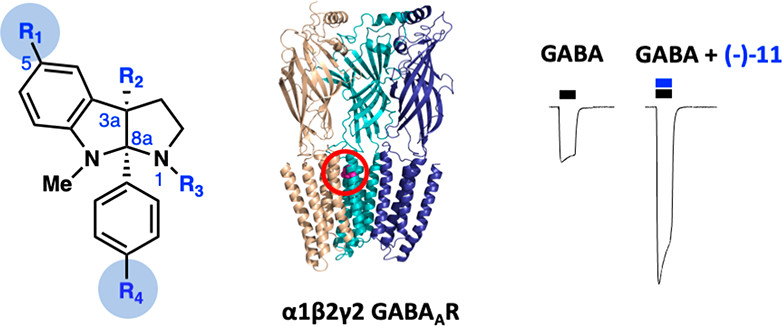
57. Synthesis and Biological Evaluation of Pyrroloindolines as Positive Allosteric Modulators of the α1β2γ2 GABAA Receptor. Blom, A. E. M.; Su, J. Y.; Repka, L. M.; Reisman, S. E.; Dougherty, D. A. ACS Med. Chem. Lett. 2020, 11, 2204. DOI: 10.1021/acsmedchemlett.0c00340
56. Synthesis of Chiral Bisoxazoline Ligands: (3aR,3a'R,8aS,8a'S)-2,2'-(cyclopropane-1,1-diyl)bis(3a,8a-dihydro-8H-indeno[1,2-d]oxazole). Hofstra, J. L.; DeLano, T. J.; Reisman, S. E. Org. Synth. 2020, 97, 172. DOI: 10.15227/orgsyn.097.0172
55. Organic Chemistry: A Call to Action for Diversity and Inclusion. Reisman, S. E.; Sarpong, R.; Sigman, M. S.; Yoon, T. P. J. Org. Chem. 2020, 85, 10287. DOI: 10.1021/acs.joc.0c01607
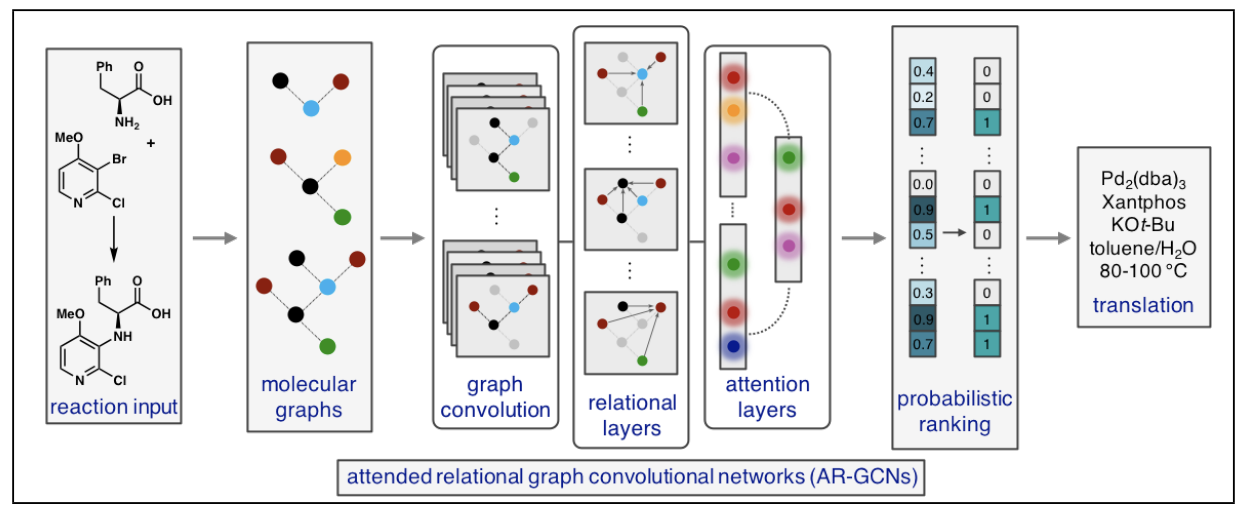
54. Graph Neural Networks for the Prediction of Substrate-Specific Organic Reaction Conditions. Ryou, S.; Maser, M. R.; Cui, A. Y.; DeLano, T. J.; Yue, Y.; Reisman, S. E. arXiv: 2007.04275 [cs.LG]. Appeared in the ICML 2020 workshop on Graph Representation Learning and Beyond (GRLB). Slides from the presentation can be found here.
53. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. Poremba, K. E.; Dibrell, S. E.; Reisman, S. E. ACS Catal. 2020, 10, 8237. DOI: 10.1021/acscatal.0c01842
52. Diversity-Oriented Enzymatic Synthesis of Cyclopropane Building Blocks. Wittmann, B. J.; Knight, A. M.; Hofstra, J. L.; Reisman, S. E.; Kan, J.; Arnold, F. H. ACS Catal. 2020, 10, 7112. DOI: 10.1021/acscatal.0c01888
51. SeO2-mediated Oxidative Transposition of Pauson–Khand Products. Dibrell, S. E.; Maser, M.; Reisman, S. E. J. Am. Chem. Soc. 2020, 142, 6483. DOI: 10.1021/jacs.9b13818
50. A 16-step synthesis of the isoryanodane diterpene (+)–perseanol. Han, A.; Tao, Y.; Reisman, S. E. Nature 2019, 573, 563. DOI: 10.1038/s41586-019-1580-x. This synthesis was featured in C&EN, Chemistry World, The Cyclo Edition, and Org. Chem. Highlights.
49. Ni-Catalyzed Conversion of Enol Triflates to Alkenyl Halides. Hofstra, J. L.; Poremba, K. E.; Shimozono, A. M.; Reisman, S. E. Angew. Chem. Int. Ed. 2019, 58, 14901-14905. DOI: 10.1002/anie.201906815
48. Enantioselective Electroreductive Coupling of Alkenyl and Benzyl Halides via Nickel Catalysis. DeLano, T. J.; Reisman, S. E. ACS Catal. 2019, 9, 6751. DOI: 10.1021/acscatal.9b01785. This method was featured in OPR&D.
47. A Modular Approach to Prepare Enantioenriched Cyclobutanes: Synthesis of (+)-Rumphellaone A. Beck, J. C.; Lacker, C. R.; Chapman, L. M.; Reisman, S. E. Chem. Sci. 2019, 10, 2315. DOI: 10.1039/C8SC05444D. This method was featured in Chemistry World.
46. Radical Deoxychlorination of Cesium Oxalates for the Synthesis of Alkyl Chlorides. Su, J. Y.; Grünenfelder, D. C.; Takeuchi, K.; Reisman, S. E. Org. Lett. 2018, 20, 4912. DOI: 10.1021/acs.orglett.8b02045
45. An Oxidative Dearomatization Approach To Prepare the Pentacyclic Core of Ryanodol. Xu, C.; Han, A.; Reisman, S. E. Org. Lett. 2018, 20, 3793. DOI: 10.1021/acs.orglett.8b01387
44. Evolution of a Strategy for the Enantioselective Total Synthesis of (+)-Psiguadial B. Chapman, L. M.; Beck, J. C.; Lacker, C. R.; Reisman, S. E. J. Org. Chem. 2018, 83, 6066. DOI: 10.1021/acs.joc.8b00728
43. Total Synthesis of (+)-Pleuromutilin. Farney, E. P.; Feng, S. S.; Schäfers, F.; Reisman, S. E. J. Am. Chem. Soc. 2018, 140, 1267. DOI: 10.1021/jacs.7b13260.
42. Synthesis of Enantioenriched Allylic Silanes via Nickel-Catalyzed Reductive Cross-Coupling. Hofstra, J. L.; Cherney, A. H.; Ordner, C. M.; Reisman, S. E. J. Am. Chem. Soc. 2018, 140, 139. DOI: 10.1021/jacs.7b11707
41. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling To Access 1,1-Diarylalkanes. Poremba, K. E.; Kadunce, N. T.; Suzuki, N.; Cherney, A. H.; Reisman, S. E. J. Am. Chem. Soc. 2017, 139, 5684. DOI: 10.1021/jacs.7b01705
40. Nickel-Catalyzed Enantioselective Cross-Coupling of N-Hydroxyphthalimide Esters with Vinyl Bromides. Suzuki, N.; Hofstra, J. L.; Poremba, K. E.; Reisman, S. E. Org. Lett. 2017, 19, 2150. DOI: 10.1021/acs.orglett.7b00793
39. Enantioselective Synthesis of (–)-Acetylapoaranotin.
Wang, H.; Regan, C. J.; Codelli, J. A.; Romanato, P.; Puchlopek-Dermenci, A. L. A.; Reisman, S. E.
Org. Lett. 2017, 19, 1698.
DOI: 10.1021/acs.orglett.7b00418
38. Chemical Synthesis of (+)-Ryanodine and (+)-20-Deoxyspiganthine. Xu, C.; Han, A..; Virgil, S. C.; Reisman, S. E. ACS Cent. Sci. 2017, 3, 278. DOI: 10.1021/acscentsci.6b00361
37. A Mild and General Larock Indolization Protocol for the Preparation of Unnatural Tryptophans. Chuang, K. V.; Kieffer, M. E.; Reisman, S. E. Org. Lett. 2016, 18, 4750. DOI: 10.1021/acs.orglett.6b02477
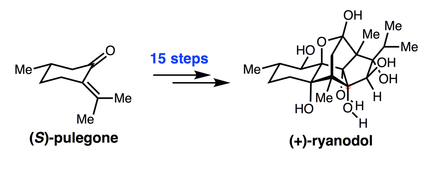
36. A 15-step synthesis of (+)-ryanodol. Chuang, K. V.; Xu, C.; Reisman, S. E. Science. 2016, 353, 912. DOI: 10.1126/science.aag1028. This synthesis was featured in C&EN and Forbes.
35. Enantioselective Total Synthesis of (+)-Psiguadial B. Chapman, L. M.; Beck, J. C.; Wu, L.; Reisman, S. E. J. Am. Chem. Soc. 2016, 138, 9803. DOI: 10.1021/jacs.6b07229. This synthesis was featured in an August C&E News article, Chem. Eng. News 2016, 94, 9, and Synfacts 2016, 12, 1003.
34. Synthesis of Enantioenriched Indolines by a Conjugate Addition/Asymmetric Protonation/Aza-Prins Cascade Reaction. Daniels, B. E.; Ni, J.; Reisman, S. E. Angew. Chem. Int. Ed. 2016, 55, 3398. DOI: 10.1002/anie.201510972
33. Enantioselective and Enantiospecific Transition-Metal-Catalyzed Cross-Coupling Reactions of Organometallic Reagents to Construct C–C Bonds. Cherney, A. H.; Kadunce, N. T.; Reisman, S. E. Chem. Rev. 2015, 115, 9587. DOI: 10.1021/acs.chemrev.5b00162
32. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling Between Heteroaryl Iodides and α-Chloronitriles. Kadunce, N. T.; Reisman, S. E. J. Am. Chem. Soc. 2015, 137, 10480. DOI: 10.1021/jacs.5b06466.
31. A Cationic Cysteine-Hydrazide as an Enrichment Tool for the Mass Spectrometric Characterization of Bacterial Free Oligosaccharides. Jang, K.-S.; Nani, R. R.; Kalli, A.; Levin, S.; Müller, A.; Hess, S.; Reisman, S. E.; Clemons, W. M. Jr. Anal. Bioanal. Chem. 2015, 407, 6181. DOI: 10.1007/s00216-015-8798-8.
30. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling Between Vinyl and Benzyl Electrophiles. Cherney, A. H.; Reisman, S. E. J. Am. Chem. Soc. 2014, 136, 14365. DOI: 10.1021/ja508067c.
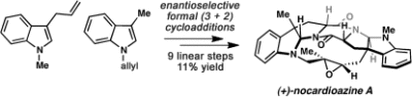
29. Enantioselective Total Synthesis of (–)-Lansai B and (+)-Nocardioazines A and B. Wang, H.; Reisman, S. E. Angew. Chem. Int. Ed. 2014, 53, 6206. DOI: 10.1002/anie.201402571. This synthesis was featured in a September 2014 post on The Offset, which can be found here.
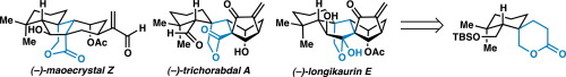
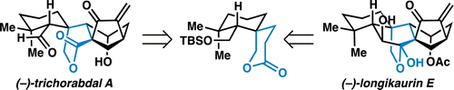
28. A Unified Strategy for the Synthesis of (–)-Maoecrystal Z, (–)-Trichorabdal A, and (–)-Longikaurin E. Yeoman, J. T. S.; Cha, J. Y.; Mak, V. M.; Reisman, S. E. Tetrahedron. 2014, 70, 4070. DOI: 10.1016/j.tet.2014.03.071.
27. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Hsiao, E. Y.; McBride, S. W.; Hsien, S.; Sharon, G.; Hyde, E. R.; McCue, T.; Codelli, J. A.; Chow, J.; Reisman, S. E.; Petrosino, J. F.; Patterson, P. H.; Mazmanian, S. K. Cell. 2013, 155, 1451. DOI: 10.1016/j.cell.2013.11.024.
26. Pd-Catalyzed Fukuyama Cross-Coupling of Secondary Organozinc Reagents for the Direct Synthesis of Unsymmetrical Ketones. Cherney, A. H.; Reisman, S. E. Tetrahedron. 2013, 70, 3259. DOI: 10.1016/j.tet.2013.11.104.
25. Recent Developments in the Catalytic, Asymmetric Construction of Pyrroloindolines Bearing All-Carbon Quaternary Stereocenters. Repka, L. M.; Reisman, S. E. J. Org. Chem. 2013, 78, 12314. DOI: 10.1021/jo4017953.
24. A Unified Strategy to ent-Kauranoid Natural Products: Total Syntheses of (–)-Trichorabdal A and (–)-Longikaurin E. Yeoman, J. T. S.; Mak, V. W.; Reisman, S. E. J. Am. Chem. Soc. 2013, 135, 11764. DOI: 10.1021/ja406599a.
23. Catalytic Asymmetric Reductive Acyl Cross-Coupling: Synthesis of Enantioenriched Acyclic α,α-Disubstituted Ketones. Cherney, A. H.; Kadunce, N. T.; Reisman, S. E. J. Am. Chem. Soc. 2013, 135, 7442. DOI: 10.1021/ja402922w.
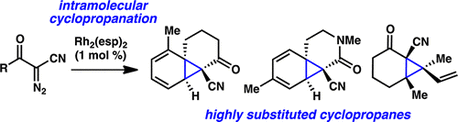
22. α-Diazo-β-Ketonitriles: Uniquely Reactive Substrates for Arene and Alkene Cyclopropanation. Nani, R. R.; Reisman, S. E. J. Am. Chem. Soc. 2013, 135, 7304. DOI: 10.1021/ja401610p.
21. Direct, Enantioselective Synthesis of Pyrroloindolines and Indolines From Simple Indole Derivatives. Wang, H.; Reisman, S. E. Tetrahedron. 2013, 69, 5622. DOI: 10.1016/j.tet.2013.04.003.
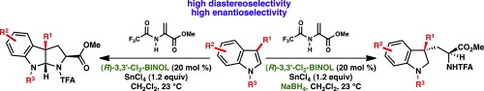
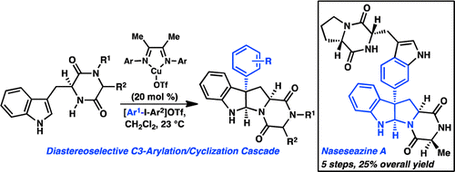
20. Copper-catalyzed Diastereoselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)-Naseseazines A and B. Kieffer, M. E.; Chuang, K. V.; Reisman, S. E. J. Am. Chem. Soc. 2013, 135, 5557. DOI: 10.1021/ja4023557.
19. Catalytic Asymmetric Synthesis of Highly Substituted Pyrrolizidines. Lim, A. D.; Codelli, J. A.; Reisman, S. E. Chem. Sci. 2013, 4, 650. DOI: 10.1039/C2SC21617E.
18. A Copper-Catalyzed Arylation of Tryptamines for the Direct Synthesis of Aryl Pyrroloindolines. Kieffer, M. E.; Chuang, K. V.; Reisman, S. E. Chem. Sci. 2012, 3, 3170. DOI: 10.1039/C2SC20914D.
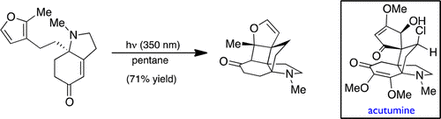
17. Rapid Construction of the Aza-Propellane Core of Acutumine via a Photochemical [2 + 2] Cycloaddition Reaction. Navarro, R.; Reisman, S. E. Org. Lett. 2012, 14, 4354. DOI: 10.1021/ol3017963.
16. Enantioselective Synthesis of Tryptophan Derivatives by a Tandem Friedel–Crafts Conjugate Addition/Asymmetric Protonation Reaction. Kieffer, M. E.; Repka, L. M.; Reisman, S. E. J. Am. Chem. Soc. 2012, 134, 5131. DOI: 10.1021/ja209390d.
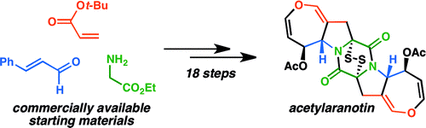
15. Enantioselective Total Synthesis of (−)-Acetylaranotin, a Dihydrooxepine Epidithiodiketopiperazine. Codelli, J. A.; Puchlopek, A. L. A.; Reisman, S. E. J. Am. Chem. Soc. 2012, 134, 1930. DOI: 10.1021/ja209354e. This synthesis was featured in November C&E News Article, found here.
14. Buchner and Beyond: Arene Cyclopropanation as Applied to Natural Product Total Synthesis. Reisman, S. E.; Nani, R. R.; Levin, S. Synlett. 2011, 17, 2437. DOI: 10.1055/s-0031-128952.
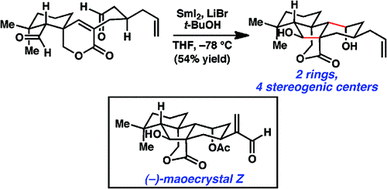
13. A Concise Total Synthesis of (–)-Maoecrystal Z. Cha, J. Y.; Yeoman, J. T. S.; Reisman, S. E. J. Am. Chem. Soc. 2011, 133, 14965. DOI: 10.1021/ja2073356. This synthesis was featured in a September 2011 post on Totally Synthetic, found here, and featured in Synfacts 2011, 12, 1271, which can be found here.
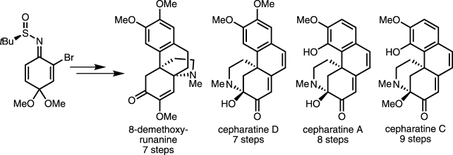
12. Short, Enantioselective Total Syntheses of (–)-8-Demethoxyrunanine and (–)-Cepharatines A, C, and D. Chuang, K. V.; Navarro, R.; Reisman, S. E. Angew. Chem. Int. Ed. 2011, 50, 9447. DOI: 10.1002/anie.201104487. This synthesis was featured in Synfacts. 2012, 8, 14, which can be found here.
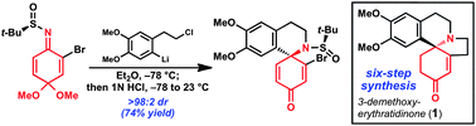
11. Benzoquinone-Derived Sulfinyl Imines as Versatile Intermediates for Alkaloid Synthesis: Total Synthesis of (–)-3-Demethoxyerythratidinone. Chuang, K. V.; Navarro, R.; Reisman, S. E. Chem. Sci. 2011, 2 (6), 1086. DOI: 10.1039/c1sc00095k.
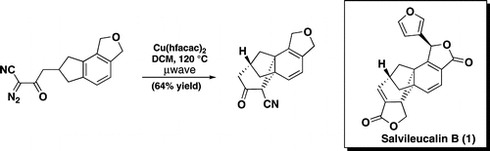
10. Enantioselective Total Synthesis of (+)-Salvileucalin B. Levin, S.; Nani, R. R.; Reisman, S. E. J. Am. Chem. Soc. 2011, 133, 774. DOI: 10.1021/ja110192b. This synthesis was featured in the February 2011 issue of Chemistry World, found here, as well as Synfacts. 2011, 6, 590, which can be found on the Synfacts page here.
9. Enantioselective Synthesis of Pyrroloindolines by a Formal [3+2] Cycloaddition Reaction. Repka, L. M.; Ni, J.; Reisman, S. E. J. Am. Chem. Soc. 2010, 132, 14418. DOI: 10.1021/ja107328g.
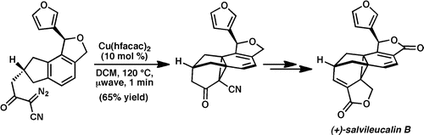
8. Rapid Assembly of the Salvileucalin B Norcaradiene Core. Levin, S.; Nani, R. R.; Reisman, S. E. Org. Lett. 2010, 12, 780. DOI: 10.1021/ol902848k.
Graduate and Postdoctoral Research
7. Welwitindolinone C Synthetic Studies. Construction of the Welwitindolinone Carbon Skeleton Via a Transannular Nitrone Cycloaddition. Freeman, D. B.; Holubec, A. A.; Weiss, M. W.; Dixon, J. A.; Kakefuda, A.; Ohtsuka, M.; Inoue, M.; Vaswani, R. G.; Ohki, H.; Doan, B. D.; Reisman, S. E.; Stoltz, B. M.; Day, J. J.; Tao, R. N.; Dieterich, N. A.; Wood. J. L. Tetrahedron. 2010, 66, 6647. DOI: 10.1016/j.tet.2010.04.131
6. Enantioselective Thiourea-Catalyzed Additions to Oxocarbenium Ions. Reisman, S. E.; Doyle, A. G.; Jacobsen, E. N. J. Am. Chem. Soc. 2008, 130, 7198. DOI: 10.1021/ja801514m
5. Evolution of a Synthetic Strategy: Total Synthesis of (±) Welwitindolinone A Isonitrile. Reisman, S. E.; Ready, J. M.; Weiss, M. M.; Hasuoka, A.; Tamaki, K.; Ovaska, T. V.; Wood, J. L. J. Am. Chem. Soc. 2008, 130, 2087. DOI: 10.1021/ja076663z
4. Total Synthesis of (±) Welwitindolinone A Isonitrile. Reisman, S. E.; Ready, J. M.; Hasuoka, A.; Smith, C. J.; Wood, J. L. J. Am. Chem. Soc. 2006, 128, 1448. DOI: 10.1021/ja057640s
3. A Mild and Efficient Synthesis of Oxindoles: Progress toward the Synthesis of Welwitindolinone A Isonitrile. Ready, J. M.; Reisman, S. E.; Hirata, M.; Weiss, M. M.; Tamaki, K.; Ovaska, T. V.; Wood, J. L. Angew. Chem., Int. Ed. 2004, 43, 1270. DOI: 10.1002/anie.200353282
2. Facile Approach to the Bicyclo[5.3.0]Decane Ring System; Efficient Synthesis of (±)-7-epi-b-Bulnesene. Kumar, J. S. R.; O'Sullivan, M. F.; Reisman, S. E.; Hulford, C. A.; Ovaska, T. V. Tetrahedron Lett. 2002, 43, 1939. DOI: 10.1016/S0040-4039(02)00171-5
1. Facile Entry to the Tetracyclic 5-7-6-3 Tigliane Ring System. Ovaska, T. V.; Reisman, S. E.; Flynn, M. A. Org. Lett. 2001, 3, 115. DOI: 10.1021/ol006823a
7. Welwitindolinone C Synthetic Studies. Construction of the Welwitindolinone Carbon Skeleton Via a Transannular Nitrone Cycloaddition. Freeman, D. B.; Holubec, A. A.; Weiss, M. W.; Dixon, J. A.; Kakefuda, A.; Ohtsuka, M.; Inoue, M.; Vaswani, R. G.; Ohki, H.; Doan, B. D.; Reisman, S. E.; Stoltz, B. M.; Day, J. J.; Tao, R. N.; Dieterich, N. A.; Wood. J. L. Tetrahedron. 2010, 66, 6647. DOI: 10.1016/j.tet.2010.04.131
6. Enantioselective Thiourea-Catalyzed Additions to Oxocarbenium Ions. Reisman, S. E.; Doyle, A. G.; Jacobsen, E. N. J. Am. Chem. Soc. 2008, 130, 7198. DOI: 10.1021/ja801514m
5. Evolution of a Synthetic Strategy: Total Synthesis of (±) Welwitindolinone A Isonitrile. Reisman, S. E.; Ready, J. M.; Weiss, M. M.; Hasuoka, A.; Tamaki, K.; Ovaska, T. V.; Wood, J. L. J. Am. Chem. Soc. 2008, 130, 2087. DOI: 10.1021/ja076663z
4. Total Synthesis of (±) Welwitindolinone A Isonitrile. Reisman, S. E.; Ready, J. M.; Hasuoka, A.; Smith, C. J.; Wood, J. L. J. Am. Chem. Soc. 2006, 128, 1448. DOI: 10.1021/ja057640s
3. A Mild and Efficient Synthesis of Oxindoles: Progress toward the Synthesis of Welwitindolinone A Isonitrile. Ready, J. M.; Reisman, S. E.; Hirata, M.; Weiss, M. M.; Tamaki, K.; Ovaska, T. V.; Wood, J. L. Angew. Chem., Int. Ed. 2004, 43, 1270. DOI: 10.1002/anie.200353282
2. Facile Approach to the Bicyclo[5.3.0]Decane Ring System; Efficient Synthesis of (±)-7-epi-b-Bulnesene. Kumar, J. S. R.; O'Sullivan, M. F.; Reisman, S. E.; Hulford, C. A.; Ovaska, T. V. Tetrahedron Lett. 2002, 43, 1939. DOI: 10.1016/S0040-4039(02)00171-5
1. Facile Entry to the Tetracyclic 5-7-6-3 Tigliane Ring System. Ovaska, T. V.; Reisman, S. E.; Flynn, M. A. Org. Lett. 2001, 3, 115. DOI: 10.1021/ol006823a











 XEC.jpeg)
 EChem Prep.jpeg)